ISO 13485 Compliance
Our platform simplifies ISO 13485 documentation and compliance processes, making quality management more efficient for medical device teams.
Loading
Nullam dignissim, ante scelerisque the is euismod fermentum odio sem semper the is erat, a feugiat leo urna eget eros. Duis Aenean a imperdiet risus.





We Transform Quality Systems, ISO 13485 Documentation, Processes, and Compliance. Our AI-powered platform streamlines quality management for medical device developers, drastically reducing the time and resources spent on documentation.
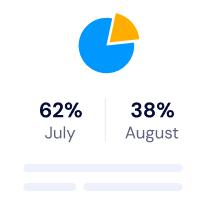
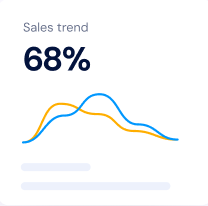
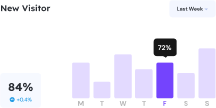
Our platform allows your team to focus on innovating and delivering while still maintaining structured quality processes. Built specifically for innovative teams establishing and maintaining effective quality systems.
Our platform simplifies ISO 13485 documentation and compliance processes, making quality management more efficient for medical device teams.
Our AI technology drastically reduces the time and resources spent on documentation, allowing your team to focus on innovation.
Our AI-powered platform streamlines quality management for medical device developers, drastically reducing the time and resources spent on documentation while ensuring compliance with industry standards.
Built specifically for innovative teams establishing and maintaining effective quality systems, igniteQuality helps you focus on what matters most - creating innovative medical devices.
Comparers
Use People
Download It


Total traffic growth of 45%
